Is Autonomous Driving coming to the UAE?
26 Apr 24
Lab ChatThe Global News Source for the World of Science and Chemicals
02 September 2021
Lab Chat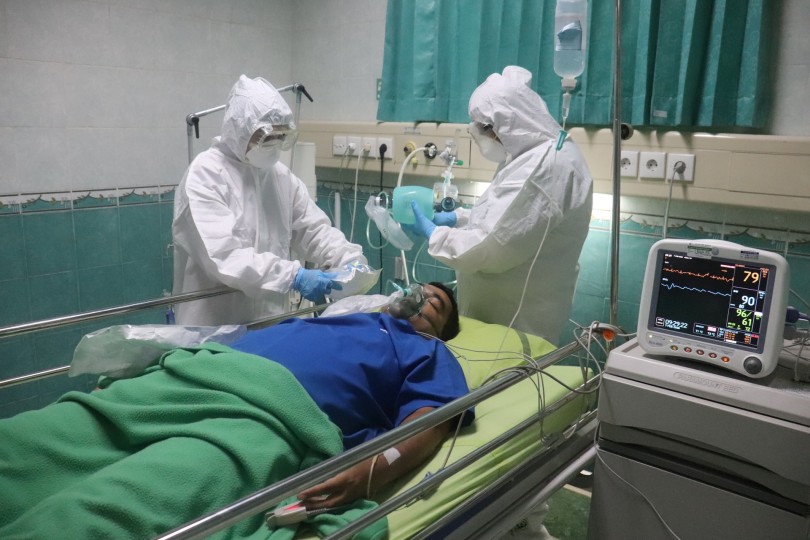
Sotrovimab, the antiviral drug produced by GlaxoSmithKline, has received very favourable results from its first initial implementation phase in the UAE. After administering the drug to some 6,000 patients over a two-week period, the UAE authorities have concluded that it is 100% effective in preventing COVID-19-related deaths.
Other early indications suggest that Sotrovimab is highly effective in mitigating the more serious symptoms of COVID-19 sufferers, including in at-risk populations. As such, it represents a very promising method of treatment going forwards. As such, the UAE has stolen a march on the rest of the world by being the first country to approve its use among the general populace.
Sotrovimab first made Emirati headlines a couple of months ago, when it was announced that the drug has been officially approved for use by the Ministry of Health and Prevention (MoHAP). Since then, 6,175 patients diagnosed with the coronavirus have been treated using Sotromovimab – and the results are extremely encouraging.
Not only does the drug have a 100% success rate in preventing death among those treated with it, but it also reduces the likelihood of a sufferer being admitted to the intensive care unit (ICU) by 99%. Moreover, 97% of those who took the drug reported a full recovery from all of their symptoms within two weeks of taking it.
To make its performance even more encouraging, the trial group to which Sotrovimab was administered contained a high proportion of at-risk candidates. More than 50% of the subjects in the study were of 50 years of age or more and already suffered from underlying health conditions, such as cancer, diabetes, heart disease or obesity. This means that they are particularly vulnerable to the effects of the virus and makes the outcome of the Sotrovimab study all the more impressive.
The fact that the UAE was the first country in the world to approve Sotrovimab for use among its general population is testament to the forward-thinking approach and advanced capabilities of the country’s medical and pharmaceutical sectors. The drug was developed by GlaxoSmithKline using sophisticated research methods which struck upon its highly promising nature.
In particular, Sotrovimab has been so effective in combatting the effects of COVID-19 due to its use of an exciting new treatment called monoclonal antibody therapies. Antibodies are the human body’s natural defences against bacteria and viruses (like the coronaviruses). They are produced by the white blood cells in our immune system as a natural defence mechanism.
Monoclonal antibodies harness this phenomenon by extracting the white blood cells and subjecting them to exposure to a targeted protein – such as the COVID-19 virus. After a lengthy process of trial and error, the monoclonal antibodies found to be most effective in vanquishing the disease can be isolated and developed into a serviceable treatment plan. In the case of Sotrovimab, they are administered via an intravenous drip, producing incredible results in those who have enjoyed its benefits so far.
DOWNLOAD PDF

2 Day Seminar Program
@ ArabLab+ 2024
24 & 25 September 2024
22 Apr 24
Lab ChatYour stay in Dubai
Labkit
Product News
Chemkit
Product News
Thinking about exhibiting at ARABLAB 2024? Watch our video to find out more.
Join the world’s leading organisations…
Join our mailing list and receive the ARABLAB newsletter and event updates.